How to fight fungal infection: the path of yeast resistance

Fungal Disease Awareness Week begins on September 18th. The week is organised by the US Centers for Disease Control and Prevention (CDC), who have chosen this year’s theme of Think Fungus. The campaign aims to highlight the growing challenges, complexities, and the devastating impact of fungal diseases.
Fungi may not be at the forefront of people’s minds when they think of infectious threats, but disease-causing fungi have the potential to devastate or disrupt human health, wildlife, natural ecosystems, food security, and global trade policies.
Annually, serious fungal diseases affect over 300 million people across the globe and are more commonly fatal than malaria and tuberculosis. People with weakened immune systems are particularly at risk, and our ability to treat patients is being hampered by increasing levels of drug-resistant strains.
Fungal infections can also wipe out animal populations and threaten our food security. Take for example the fungal disease chytridiomycosis, which is responsible for the mass extinction of amphibian species over the past few decades, or Magnaporthe oryzae, the fungal pathogen behind the wheat blast outbreak that wiped out harvests in Bangladesh. Worryingly, M. oryzae and other fungal blights of crops continue to spread across the globe.
To mark the week and the importance of fungal research, the Institute of Infection spoke with Professor Darius Armstrong-James, Professor Mat Fisher, and scientists representing the breadth of fungal research in Imperial College London, to highlight how Imperial is working to address critical fungal challenges, including through Imperial’s Fungal Science Network.
Aspergillus in the clinic

Professor Darius Armstrong-James is a clinician and scientist who specialises in understanding the mechanisms by which complex pathogens such as fungi that are difficult to diagnose and treat cause such devastating diseases. He leads the Royal Brompton Hospital fungal diseases service, which provides clinical and diagnostic support for patients with fungal disease, and his research programme in the Department of Infectious Disease focuses on aspergillosis and how it interacts with the immune system.
He leads a UK Cystic Fibrosis (CF) Trust Strategic Research Centre called TrIFIC (Targeting Immunotherapy for Fungal Infections in Cystic Fibrosis) which focuses on investigating whether immunotherapies are useful during Aspergillus infection and which individuals are most likely to benefit from them. Aspergillus fumigatus is found in the phlegm of 50% of people with CF by the time they reach adulthood. One-third of these individuals go on to develop Aspergillus bronchitis, a severe airway infection, and 15% experience allergic broncho-pulmonary aspergillosis. These problems can lead to lung inflammation and a decline in their function and stop patients from being eligible for a lung transplant.
Some fungal infections are also resistant to anti-fungal medications, which is leading researchers like Professor Armstrong-James to look to immunotherapies as potential treatment options.
[Immunotherapies] are of great potential for infectious diseases, as shown in COVID-19, because of the evolving race that occurs between pathogens and their host.
Immunotherapies have the potential to avoid antimicrobial resistance and can be very helpful when the immune system is weak or overactive. They have already been used successfully in patients with CF and Aspergillus infection, and more are being created to target airway inflammation. The investigation will lead to better identification and more personalised treatments for problems arising from A. fumigatus infection in people with CF.
Professor Darius Armstrong-James
Professor Darius Armstrong-James
TrIFIC has 223 participants across three centres.
TrIFIC has 223 participants across three centres.
Aspergillus everywhere and the rise of resistance

Professor Mat Fisher (School of Public Health) and colleagues have been working on the emergence of resistance to antifungal drugs both in the clinic and in nature in order to understand the interplay between the environment (e.g., agriculture), amplifiers (e.g., farm or food waste disposal) and exposures (how and where humans, animals and plants encounter resistant drug-resistant fungi).
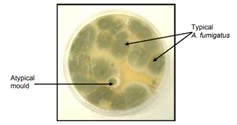
The aforementioned A. fumigatus, which can cause the disease aspergillosis in at-risk humans, is a very common mould in soils and air. Emerging resistance to antifungal drugs by this mould is an unfortunate consequence of the prolific use of fungicides to protect crops against disease.
The health threat is significant. A recent citizen science project by Professor Fisher et al. showed that more than 5% of A. fumigatus spores in the UK air are now resistant to both clinical and agricultural azole antifungals. And as we are all exposed to this mould daily, this is especially serious for people who are immunocompromised, who are at a greater risk of aspergillosis.
However, it’s not all bad: we’ve recently seen the development of two new highly promising antifungal drugs to which resistance is rare or non-existent.
Professor Mat Fisher
Professor Mat Fisher
Aspergillus fumigatus compared to atypical mould
Aspergillus fumigatus compared to atypical mould
Sporotrichosis: outbreak of the cat infection

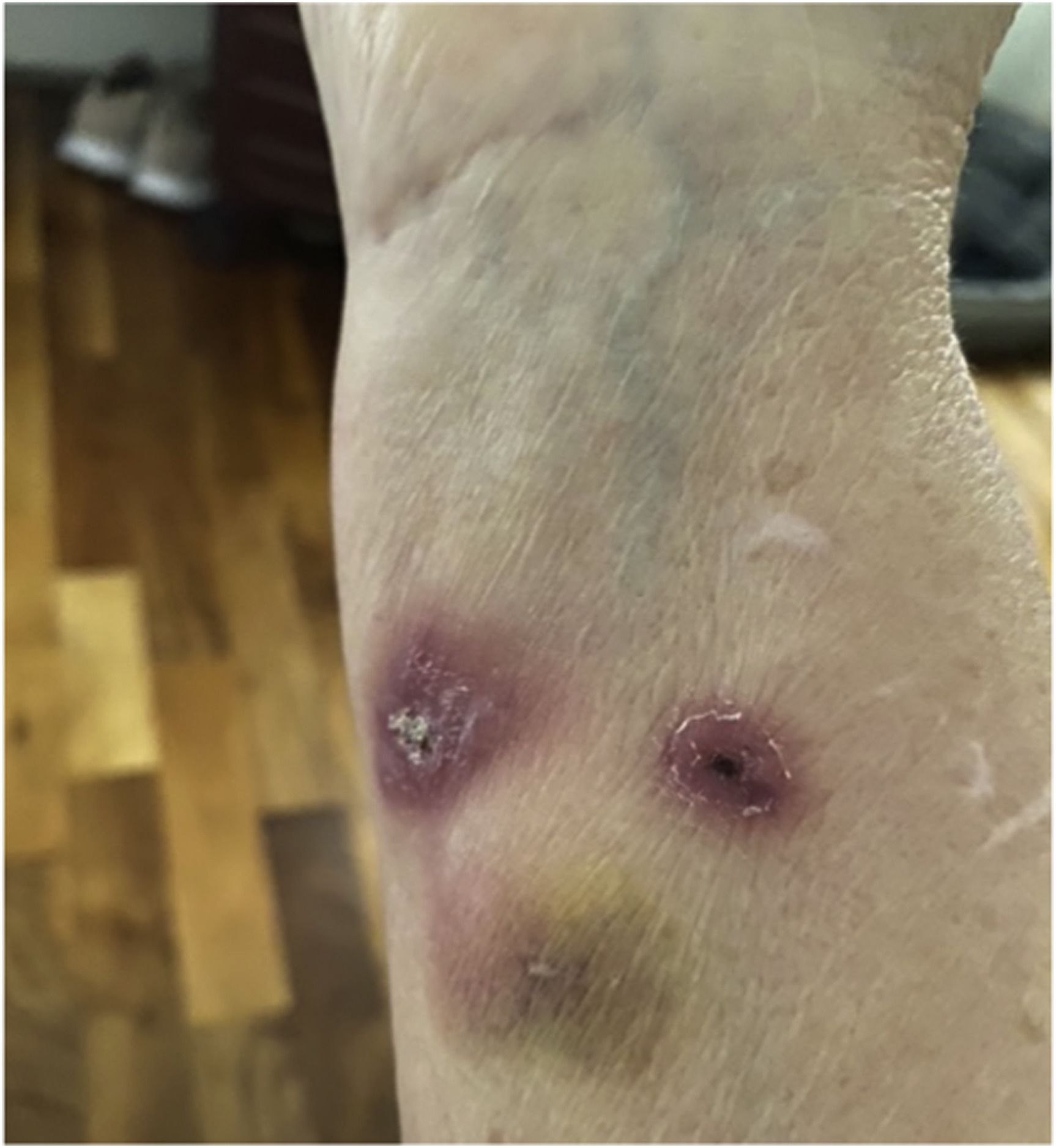
Right forearm of patient with Sporotrichosis during treament
Right forearm of patient with Sporotrichosis during treament
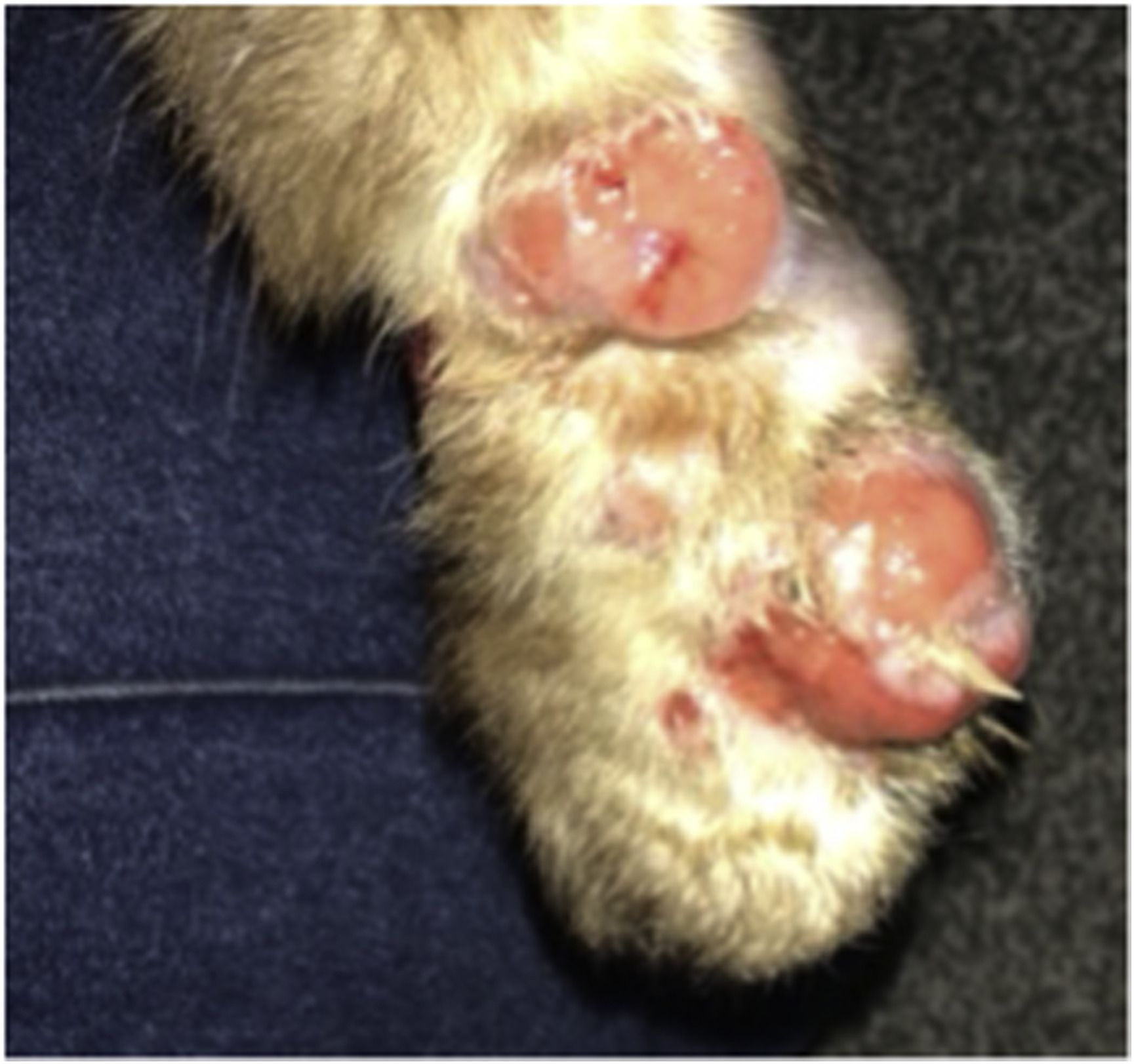
Cat paw with lesions at the beginning of treatment
Cat paw with lesions at the beginning of treatment
Professor Armstrong-James also described a recent case study he was involved with that demonstrated the threat of ‘anthropogenic’ spread of fungi, defined as spread by human activity. The infection was sporotrichosis.
Sporotrichosis is a fungal infection found mostly in plants but also in cat claws. This fungus has caused outbreaks for decades in cats and humans in Brazil, where it is spreading. If transmitted to humans (e.g., via cat scratches) it can cause serious traumatic skin infections that require several weeks of toxic antifungals, and for people with weakened immune systems or lung problems, the infection can be life-threatening.
In 2021, the first human outbreak of cat-transmitted sporotrichosis outside of Latin America occurred in North West London, which was spread by a cat in the UK that had been imported – and lived symptom-free – from Brazil two years before. Professor Armstrong-James’s expertise was engaged to contribute to the outbreak investigation.
While further spread was prevented in this case by the cat being confined indoors and treated, the incident raised concerns for future outbreak potential because the asymptomatic infection was not detected by UK import control. Antimicrobial resistance to the fungus has been reported in cats, which is concerning. The risk of spread could be worse in countries where the number of cats being imported is larger and less regulated.
Scientists consider this spread of sporotrichosis to be a slow-burning but serious global public health issue, which needs addressing. “We don’t want it circulating in our cat population,” said Professor Armstrong-James.
Anthropogenic spread is caused by trade and travel.
It is easy to imagine the impact of moving a microbe from one region into a new environment, where there are no ecological competitors or competing microbes to impede its spread, or where the immune systems of their host haven’t evolved to fight them.
Scientists are using genomics to track and trace the many fungal infections which are moving around the planet (see Dr Johanna Rhodes story below).
Professor Fisher reflected that just one mistake, e.g., from the import of one cat or one seed with a single spore, could produce devastating effects. “Fungi are good at dispersing. If we move something around without sterilising it, we will move a fungus of some description with it.”
Resistance in a highly chemicalised world

A unique aspect of the treatment of fungi is that the same antifungal agents (e.g., azoles) are used ubiquitously in healthcare (human and animal), agriculture, and even antifungal paints on ships. Such ‘dual use’ accelerates the spread of resistance across the globe and means that we are losing our first line of defence against opportunistic fungal infections in the clinic.
For patients, it means that the drugs we rely on are no longer working as well as they once did. Moreover, in agriculture, the evolution of resistance to crop-destroying fungi is a perennial threat necessitating the development of new types of fungicides. Resistance therefore threatens our food security, the environment, ecology, and human health.
We need to find system-level solutions and investments that incorporate new treatment options alongside new diagnostic approaches and clinical practices (see Dr Anand Shah below), stewardship, policy changes and biosecurity.
A critical need for global policy changes

As shown by the examples above, fungal scientists are demonstrating the critical need for major policy changes, including concerning border control, antifungal stewardship, and global trade. Government and policymakers must be made aware of the science and weigh up the health, food security, and ecological impacts against the cost and the impacts on global trade. Moreover, these are things that countries cannot do on their own: it must be a global effort, with ambitions on a global scale: see for example the global surveillance system or trade agreements described by Dr Rhodes below.
It is, therefore, no wonder that fungal experts are increasingly being included in national and international policy discussions. For example, Professor Armstrong-James’s expertise was recently sought by the Department of Health towards their five-year action AMR plan. “It’s good to see fungi on the agenda,” he said.
A Transdisciplinary Fungal Science Network with One Health at its core

Fungal Network - Summer 2023 meeting
Fungal Network - Summer 2023 meeting
An event showcasing the wealth of contributions to fungal science across our three key research themes: pathogenesis of fungal diseases, biotechnology and engineering, and ecology, evolution, and the environment.
An event showcasing the wealth of contributions to fungal science across our three key research themes: pathogenesis of fungal diseases, biotechnology and engineering, and ecology, evolution, and the environment.
In 2021, envisaging the benefits of an institution-led programme on fungi, Professor Armstrong-James obtained approval from College to establish a transdisciplinary all-encompassing fungal network. The resulting Imperial College London Fungal Science Network of Excellence brings together biologists, ecologists, immunologists, climate scientists, economists, engineers and clinicians to answer the many critical issues and challenges in fungal science. The aims of the Network include education, advocacy and developing broad and far-reaching collaborative science.
The Network’s focus isn’t just on disease-causing fungi. Our planet and lives would be unrecognisable without the benefits that fungi bring, which include their role in medicine (e.g., penicillin) and maintaining our ecosystem through the decomposition cycle. Researchers, including Dr Stanley (below) are contributing to this discovery science.
“We don’t have to look far for extraordinary questions in the world of mycology. There are so many millions of fungi out there, and so many disease problems, business opportunities, and elegant symbioses to study,” enthused Professor Fisher.
“Bringing together different minds and different disciplines is the only way that these grand challenges can be solved, and the opportunities grasped.”
For example, coalescing the breadth of expertise at Imperial College London ideally positions the Network to embrace a ‘one health’ approach. Professor Armstrong-James reflected: “[fungal science] is very much embedded within the broader biology and geography of the planet, which I think is an amazing aspect of this particular speciality.”
Despite the critical impacts, fungal disease research has been severely neglected for many years. However, this has been improving since the recent publication of the WHO fungal priority pathogen list and with improved recognition of the problem by the media and the public. Professor Armstrong-James and Colleagues are currently seeking funding to create a Transdisciplinary AMR Fungal network with collaborators across the UK. If funded, it will bring together experts in genomics, diagnostics and surveillance, medical mycology, antifungal stewardship, public outreach, regulatory control, waste management, climate change, agriculture and agrichemicals, drug discovery, social sciences, and health policy.
Bioengineering approaches to microbial interactions
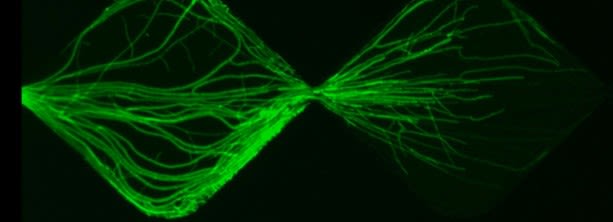
Dr Claire Stanley (Department of Bioengineering) develops novel microfluidic technologies to probe the interplay between microbes at the single cell level. Her devices consist of tiny microorganism-holding wells on either end of a ‘chip’ with connecting microscopic channels. The technology allows researchers to gain insights into biological events that could not be achieved from traditional plate methods, including imaging the movement of hyphae in real-time and the heterogeneity between individual cells.
In collaboration with microbiologists and other scientists, Dr Stanley’s devices have been adapted to answer questions about how fungi interact with bacteria, other fungi, plants, and nematode worms, giving critical insights in microbiology, environmental science, and agriculture. Dr Stanley is an ardent believer that her technology should be usable by the researchers asking the biological questions rather than specialist technologists. “Simplicity is important,” she said.
In recalling a time when she was most inspired, Dr Stanley described how an exploratory collaboration with a colleague studying microscopic nematode worms became the penny-drop moment when she realised the potential power of the technology. When the researchers combined the microbiologist’s expertise with Dr Stanley’s technology, they discovered new insights into the ways by which fungi defend themselves against nematode attack, and captured images of the fungi communicating the threat via their hyphae. The story was picked up by the New York Times.
Another collaborative project - this time with a PhD student studying agricultural biocontrol - focused on fungal-fungal interactions. Stunning real-time images (below) showed how a pathogenic fungus (Fusarium graminearum) responded when placed in proximity to a fungal biocontrol agent used on crops (Clonostachys rosea). This was another example of researchers gaining astonishing insight beyond their expectations.
Dr Stanley’s microfluidic device is also helping the study of arbuscular mycorrhizal fungi, which form important symbiotic relationships with 70-80% of land plants. It gives the researchers a tool to explore how the soil microbiome helps plants take up nutrients, how hyphae search physical space to allow the fungus to find plant roots, and how the environment (soil microbiome, pesticides, and antibiotics) influences fungal behaviour.
Fungal-fungal interaction
Fungal-fungal interaction
A One Health approach to fungal infection outbreaks

With recent advances in rapid sequencing capabilities, researching human fungal pathogens using genomic epidemiological techniques can be applied to real-time outbreaks of fungal infections. Dr Johanna Rhodes (School of Public Health) uses omics technologies to generate sequence data, which can be mined to understand drug resistance mechanisms, transmission events, and how these pathogens spread through time and space.
Over the last few years, she has become interested in where drug-resistant fungi are coming from in the environment, how resistance is evolving in a changing climate, and what impact that has on humans. She also worked with a team at the Big Data Institute at Oxford to create the first genomic surveillance platform for the fungal pathogen, Candida auris, which causes severe illness and spreads easily in healthcare facilities. She said: “We hope this surveillance platform will help clinicians determine drug resistance profiles quickly (it can analyse sequence data in just a few minutes)”.
Dr Rhodes emphasised how the ‘one health’ approach is highly relevant to fungal science because “fungi are everywhere”. It is an approach that she embraces:
- One project looks at samples from patients and the environment and uses genomics to understand how patients acquire their infections from the environment.
- Related to healthcare systems, her work has helped to implement infection control measures in hospitals (e.g. updating disinfectants) which have successfully prevented recent outbreaks of C. auris.
- Looking to food security, Dr Rhodes noted: “Fortunately, there are ways to protect crops… one way is to set up a global surveillance network using genomics (part of the WHO strategy 2022-2032) so that the movements of pathogens can be tracked. We can also improve biosecurity in traded seeds and non-sterile goods to slow the spread of fungal infections."
- Referring to the agricultural industry, for the rise in drug-resistant Aspergillus, she believes the best way to combat this is to “stop using the same drug classes clinically and agriculturally…This would require a globally agreed policy where every country accepts this.”
- And, looking to ocean science, one of her PhD students has recently partnered with Ocean Cleanup to sample ocean water to identify which drug-resistant fungi are present.
A summary of Dr Johanna Rhodes's work by her PhD student, Hugh Gifford
A summary of Dr Johanna Rhodes's work by her PhD student, Hugh Gifford
A map of Candida auris worldwide
A map of Candida auris worldwide
Dr Johanna Rhodes presenting at the 16th European Conference on Fungal Genetics
Dr Johanna Rhodes presenting at the 16th European Conference on Fungal Genetics
Pulmonary aspergillosis: preventing fungal lung infections
Dr Anand Shah
Dr Anand Shah
Cross-sectional CT
Cross-sectional CT
Dr Anand Shah (School of Public Health and Consultant Respiratory Physician at the Royal Brompton and Harefield NHS Foundation Trust) works to improve the outcomes of people suffering from lung fungal infections, such as pulmonary aspergillosis, which affects three million people worldwide and for which chronic disease has a worse prognosis than most cancers. Together with his research group, he aims to understand the immune and genetic factors that predispose people to fungal lung disease, determine how these could be targeted to improve the human immune response to fungal infection, and tackle the global emerging problem of antifungal resistance.
Invasive aspergillosis is the most commonly missed infection-related diagnosis in intensive care unit patients, but there are many ways this can be improved, for example by using novel serological and molecular diagnostics. However, there are still significant obstacles, including a lack of awareness and guideline-based recommendations not being adopted. “Hence, there is a strong role to adopt antifungal stewardship in intensive care to educate, promote best practice and improve outcomes,” said Dr Shah. More research is also needed to apply novel rapid molecular sequencing approaches to fungal diagnosis and integrate this with the immune response.
Dr Shah is also exploring the uses of machine learning and artificial intelligence throughout clinical medicine, which can be used to unlock unstructured electronic health records, understand disease phenotypes, identify patients for stewardship intervention, analyse cross-sectional radiology imaging to evaluate treatment response in fungal disease, and integrate complex datasets.
One of Dr Shah’s current research projects, FREAL (Fungal resistance evolution and acquisition in chronic lung disease), aims to understand why and how individuals with chronic fungal disease develop antifungal resistance to triazoles (currently the only orally available antifungal) and whether the resistance results from mutations from environmental agricultural triazole use or from exposure to antifungal medication. Dr Shah’s group also aims to determine whether this resistant strain is transmitted from home or hospital bed environments. His group is doing this by performing genomic analysis of the Aspergillus in phlegm from individuals with chronic fungal lung disease at different moments in time and studying the air and soil samples from their environment.
I am very lucky to work in a close network of fungal researchers who are committed to improve outcomes in fungal lung disease and particularly anto-microbial resistance.”